New CardioRNA Position paper: Peripheral blood RNA biomarkers for cardiovascular disease
Very happy to share the latest EU-CardioRNA position paper out now in Cardiovascular Research. Congratulations to Maarten Vanhaverbeke, Yvan Devaux and all co-authors.
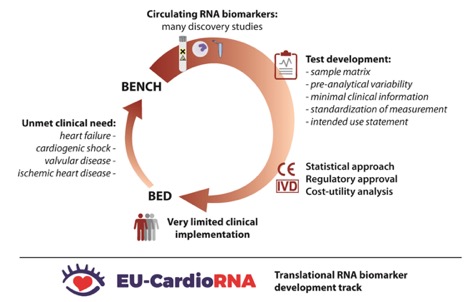
A practical how-to and bench-to-bedside guide study RNA biomarkers in CVD.
Access the PDF here: Cvab327
Access articleAbstract: Despite significant advances in the diagnosis and treatment of cardiovascular diseases, recent 4 calls have emphasized the unmet need to improve precision-based approaches in cardiovascular 5 disease. Although some studies provide preliminary evidence of the diagnostic and prognostic 6 potential of circulating coding and non-coding RNAs, the complex RNA biology and lack of 7 standardization have hampered the translation of these markers into clinical practice. In this 8 position paper of the CardioRNA COST action CA17129, we provide recommendations to 9 standardize the RNA development process in order to catalyze efforts to investigate novel 10 RNAs for clinical use. We list the unmet clinical needs in cardiovascular disease, such as the 11 identification of high-risk patients with ischemic heart disease or heart failure who require more 12 intensive therapies. The advantages and pitfalls of the different sample types, including RNAs 13 from plasma, extracellular vesicles and whole blood, are discussed in the sample matrix, 14 together with their respective analytical methods. The effect of patient demographics and highly 15 prevalent comorbidities, such as metabolic disorders, on the expression of the candidate RNA 16 is presented and should be reported in biomarker studies. We discuss the statistical and 17 regulatory aspects to translate a candidate RNA from a research-use only assay to an in-vitro 18 diagnostic test for clinical use. Optimal planning of this development track is required, with 19 input from the researcher, statistician, industry and regulatory partners.
